About Herapin Therapeutics
Welcome to Heparin Therapeutics, where cutting-edge medical research meets unparalleled dedication to improving patient outcomes. As pioneers in the field of heparin and inhalation-administered drugs, we are committed to advancing healthcare through innovation, integrity, and excellence.
Our team of world-class scientists and researchers specialize in the development and optimization of heparin-based therapies, pushing the boundaries of medical science to offer groundbreaking solutions for a range of medical conditions. With a focus on inhalation administration, we aim to develop safe and highly differentiated pulmonary treatments for acute and chronic respiratory diseases to that enhance patient comfort and compliance.
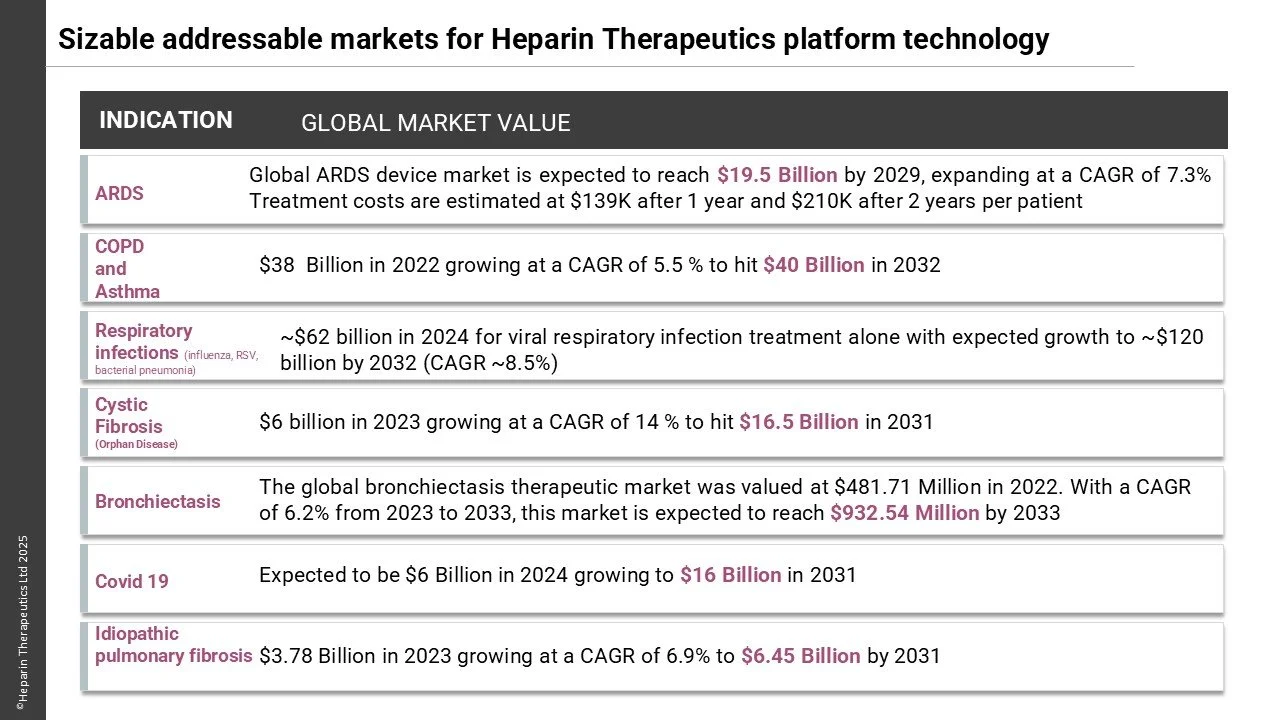
There is a significant unmet need for treatments aimed at reducing lung inflammation in both ICU and outpatient settings. Prolonged time on ventilators is associated with poor outcomes, underscoring the necessity for effective therapies.
The efficacy and safety of Unfractionated Heparin in the treatment of respiratory disease has been demonstrated through more than 50 clinical studies involving our founders investigating the off-label nebulisation of commercially available of heparin injections in respiratory disease. However, despite the impressive clinical outcomes such an approach results in sub-optimal suboptimal lung delivery and retention and is impractical for routine clinical use and so currently, no approved inhaled UFH product exists.
Heparin Therapeutics is pioneering HT-231, the first patented, safe, and well- tolerated unfractionated heparin (UFH) nebulized medicine utilizing our proprietary drug delivery platform, for the treatment of Acute Respiratory Distress Syndrome (ARDS). This PoC data is expected to be translatable to other prevalent lung diseases including Chronic Obstructive Pulmonary disease (COPD), Asthma, Idiopathic Pulmonary Fibrosis (IPF) and Bronchiectasis.
Founders
-

Professor Frank van Haren (CMO)
MD PhD EDIC FCICM PGDipEcho
Frank van Haren is Professor at the Australian National University’s College of Health and Medicine and Director of the Intensive Care Unit at St George's Hospital in Sydney, Australia. He is also Conjoint Professor at the University of New South Wales, Adjunct Professor at the University of Canberra, and honorary Professorial Fellow at the George Institute for Global Health.
He is an established international leader and expert in clinical and translational research. He has led 2 investigator-initiated multi-national collaborative COVID-19 studies investigating inhaled heparin. He has published over 160 articles and book chapters and given over 140 invited presentations at international scientific conferences.
-

Prof. Clive Page (CSO)
OBE, BSc, PhD, FSoB, FBPhS
Clive Page is a member of the Scientific Advisory Committee at MedPharm and Professor of Pharmacology at King’s College London. He is Co-Founder and previous Chairman of the Board of Verona Pharma plc, a NASDAQ listed company developing new drugs for the treatment of respiratory diseases and has held Non- Executive Board positions at Stirling Products Ltd, Revolo (formerly Immune Regulation Ltd) and Babraham Biotechnology Ltd. Professor Page was awarded an OBE for “Services to Pharmacology” in 2017 and has recently served as a Non-Executive Director of PreP Biopharma and as Non-Executive Chairman of the Board of EpiEndo. Clive is also Chairman of the Board of Trustees of the Fraunhofer Institute of Experimental Medicine and Toxicology in Hannover and the William Harvey Research Foundation in the UK. Recently, a company he founded, Verona was acquired by Meck for $10 billion.
-

Prof. Marc Brown (CEO)
BSc PhD CChem FRSC
Marc Brown co-founded MedPharm in August 1999. He has been the guiding force behind all of MedPharm’s scientific developments and intellectual property. He has been Professor of Pharmaceutics and Toxicology in the School of Pharmacy, University of Hertfordshire since 2006 and has visiting/honorary professorships at the Universities of Reading and King’s College London. He has over 200 publications and 30 patents describing his work. His research interests lie mainly in drug delivery to the skin, nail and airways. To date, he has been involved in the pharmaceutical development of over 80 products that are now on the market in Europe, America and Japan. He is a scientific advisor in formulation development for numerous pharma companies around the world and was recently involved in the $80 million Series A investment in Mosanna where he was CSO.
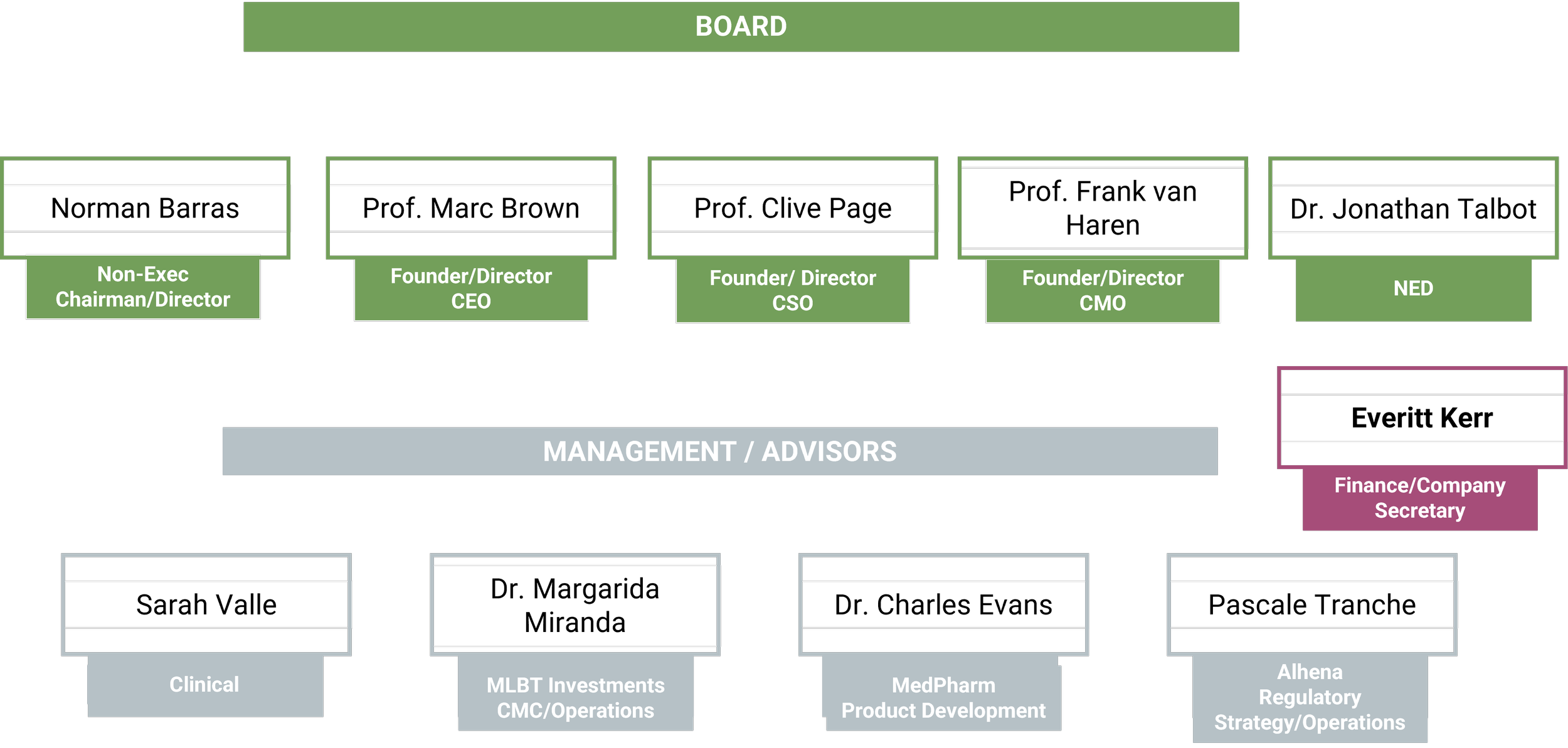
Company structure
Management/Advisors
-
Norman Barras
Non-Executive Chairman
Norman Barras has an BSc. in Chemistry from the University of the West of Scotland and an MSc. in Biochemical Pharmacology from the University of Hertfordshire. Mr. Barras has over forty years of experience in the Pharmaceutical Industry, working at, Vice President/Director level in; Pharmaceutical Research; Product Development; Manufacturing; International Registration and Business Development of drug products. He has held Senior positions at: Patheon, Vice President Global Clinical Manufacturing (USA, Canada, Europe and Asia Pacific); Lonza Biologics, Managing Director (Slough); Norgine B.V. Vice President Pharmaceutical Research (USA and Europe) and Managing Director PCI Pharma Services (formerly Penn Pharma). With extensive knowledge of taking drugs from concept all the way to market and with expertise in dealing with Global Regulatory Agencies such as the FDA, MHRA, EMA among others. A member of the Scientific Advisory Committee at MedPharm as well as holding a number of Board positions with both Pharmaceutical Research, Manufacturing and Pharmaceutical Packaging Companies.
-
Sarah Vale
Clinical BMeDSc, BN
She is co-founder and major shareholder of an Australian private entity, Mucpharm Pty Ltd, developing targeted therapeutics in oncology, infection and respiratory conditions. Through establishing and growing the company, Sarah now holds Chief Operating Officer responsibilities and coordinates the scientific and clinical development programs, managing 14 senior scientists. She is familiar with private and publicly listed requirements of business in Australia and business development in the USA. Sarah has more than 10 years preclinical research and clinical trial experience in both new drug, biologics and device developments. She manages regulatory US FDA and EMA submissions (orphans, INDs, protocol advice, scientific assistance Sarah is an inventor on several patents including new drug and device patents. She has coordinated capital raise of more than USD $65 million over a three year period and two out-licensing (upfront, milestone and royalty) deals surrounding distribution and sales rights.
-
Dr Jonathan Talbot
(Non-Executive Director) MA PhD
Dr. Jonathan Talbot is an experienced biotech executive with more than 15 years in the pharmaceutical industry with expertise in strategy, clinical development and business development. He is currently CEO of Melodia Therapeutics and prior to that he was the Co-Founding CEO of Mosanna Therapeutics, where he led the company through multiple financing rounds exceeding $85 million and advanced a novel nasal spray therapy for obstructive sleep apnea through preclinical development to MHRA approval for entry into clinical trials. Jonathan spent nearly a decade at Roche, where he served in a broad range of roles. As Global Scientific Director he drove medical aspects of the clinical development, successful launch and marketing of several novel biologic therapies. In Pharma Partnering he gained deep experience in deal making and valuation, contributing to numerous licensing and acquisition deals. Jonathan holds a PhD in Pharmacy from King’s College London and a Master’s in Finance from ESCP Business School, bringing a unique blend of scientific and financial expertise to company building and investor engagement.
-
Dr. Margarida Miranda
MLBT Investments CMC/Ops, Pharm.D., Ph.D.
Margarida Miranda graduated in Pharmaceutical Sciences (University of Lisbon) and completed her PhD in Pharmaceutical Technology (University of Coimbra, Portugal) and has published 18 papers, 4 book chapters and edited 1 book on the field of topical drug delivery. After completing her Ph.D she translated her research into the R and D department of Laboratórios Basi, where she has implemented IVRT/IVPT methods within a Quality Management System. In addition, she actively contributed to the definition of strategies to demonstrate topical bioequivalence, in accordance with the current European and American guidelines and has been involved in the development, submission and approval of several topical generic products in the European market. Whilst consulting for MLBT she is also Assistant Professor of Pharmaceutical Technology, as well as Cosmetic Science, at Egas Moniz School of Health and Science.
-
Dr. Charles Evans
MedPharm Product Development, BSc PhD
Senior Vice President of Pharmaceutical Development of MedPharm MedPharm is an end-to-end CDMO specializing exclusively in topical and transepithelial drug products, providing comprehensive support from early-phase research through to clinical and commercial manufacturing. MedPharm’s expertise spans delivery to the skin, lung, nose, eye, ear, nail as well as other mucosal membranes. MedPharm's unique approach to product development focuses on de-risking the development process using thorough pre-formulation and formulation development approaches before employing industry-leading in vitro models and process development capabilities. This approach has supported the approval of more than 80 marketed products across the USA, Europe and Asia. Dr. Charles Evans serves as Senior Vice President of Pharmaceutical Development at MedPharm, with more than 20 years of experience in advancing products from early-phase through to commercialisation across topical, inhalation, transdermal, and injectable platforms. He has particular expertise in complex delivery systems designed to optimise drug efficacy, quality, and safety.Throughout his career, Dr. Evans has led multidisciplinary teams in the design and optimisation of a broad range of dosage forms, including liquids, semi-solids, aerosols, and transdermal patches. He played a pivotal role in developing MedPharm’s proprietary MedSpray® technology, now licensed to enhance client product performance. In his current role, he oversees global product development strategy and leads MedPharm’s UK centre of excellence for formulation development. Dr. Evans drives the delivery of robust, effective drug products, covering the full development lifecycle—from early formulation to scale-up and manufacturing—supported by a strong foundation in regulatory compliance and scientific rigour. His leadership helped establish MedPharm’s reputation for excellence in topical and transdermal drug delivery. He continues to contribute to the field through publications, patents, and active industry engagement.
-
Pascale Tranche
Alhena Regulatory Strategy/Operations, BSc PhD
Pascale has extensive experience in pharmaceutical and drug development, as well as in project management, gained through nearly 20 years in the pharmaceutical industry in both the USA and France. She began her career at Pentech Pharmaceuticals, a biotechnology company based near Chicago (USA), where she first served as Laboratory Manager and then Project Manager. During this time, she acquired hands-on experience in CMC analytical development, formulation, technology transfer to CMOs, FDA interactions, and GMP operations. Pascale then joined Galderma (France) as Manager of Pharmaceutical Activities, where she expanded her expertise in dermatological products, regulatory interactions (as CMC representative), and the operations of a large international organization. In 2008, Pascale moved to Nicox as Project Manager. She initially oversaw pharmaceutical development activities before taking on the management of global development programs. In this role, she led multidisciplinary project teams, reporting to executive management and the board. Her experience broadened to include full development lifecycle management, ophthalmology, medical devices, and sterile products. After almost two decades in the pharmaceutical industry, Pascale transitioned to consultancy and co-founded Alhena Consult in 2019 with three other partners.
Partners